About GETTING APPROVAL
Understanding the intricacies of the pharmaceutical and biotech markets is crucial for global expansion. In this context, obtaining regulatory approval in Japan stands as a strategic linchpin for our endeavors.
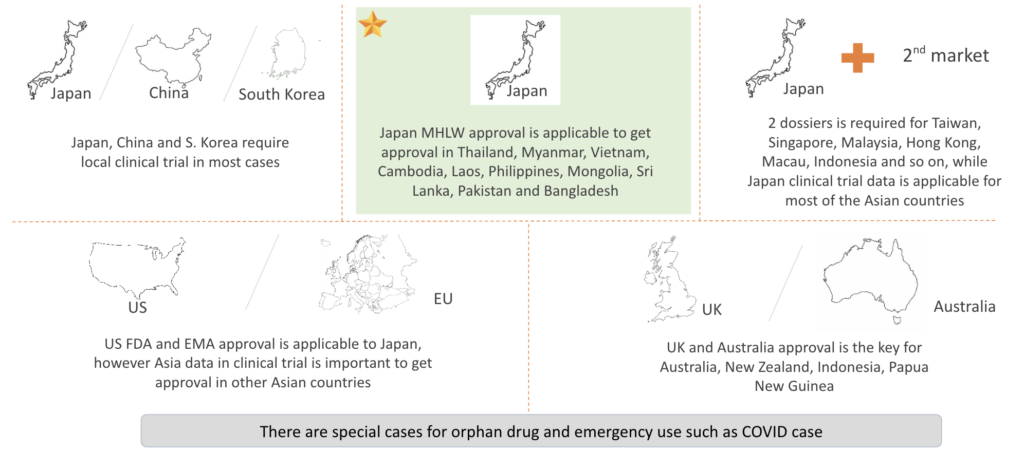
Recognized for its stringent and highly respected evaluation criteria, the Japanese MHLW’s endorsement not only fortifies our presence in the home market but remarkably streamlines our expansion into numerous Asian countries. From Taiwan to Thailand and from Myanmar to Mongolia, Japan’s clinical trial data and regulatory approval resonate with credibility, allowing a swift and smooth transition into these markets.
By targeting local clinical trials in key areas like China and South Korea, we further solidify our global footprint. With a keen eye on international guidelines and nuances, our aim is not just to enter markets but to establish a trusted presence, ensuring our innovative therapies reach as many patients as possible.